Research
Lipid Microarrays:
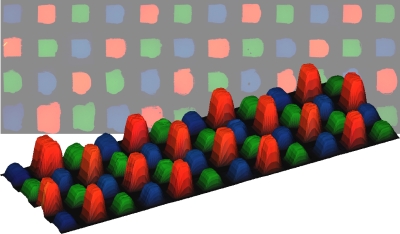 There is an increasing interest in the development of microarray based assays for
high-throughput analysis and detection of biomolecules such as DNA and proteins. Array-based
analyses have proven extremely useful for DNA detection and sequencing. The general
versatility of microarrays for multi-variable, high-throughput analysis has also been
applied to solve analytical problems in the emerging field of proteomics. One area
of chemical and biological analysis which could potentially benefit from the use of
microarrays is the study of biological membranes. For example, the ability to form
stable lipid membrane arrays would be useful for studies on membrane associated proteins,
which account for up to two-thirds of known drug targets.
There is an increasing interest in the development of microarray based assays for
high-throughput analysis and detection of biomolecules such as DNA and proteins. Array-based
analyses have proven extremely useful for DNA detection and sequencing. The general
versatility of microarrays for multi-variable, high-throughput analysis has also been
applied to solve analytical problems in the emerging field of proteomics. One area
of chemical and biological analysis which could potentially benefit from the use of
microarrays is the study of biological membranes. For example, the ability to form
stable lipid membrane arrays would be useful for studies on membrane associated proteins,
which account for up to two-thirds of known drug targets.
Microfluidics has proven to be a promising method for producing patterned lipid bilayers; however, the 2D nature of these devices limits the addressable elements in an array to linear channels on the surface. We have developed a means to create multicomponent lipid bilayer arrays using a 3D continuous flow microspotter (CFM) system developed in the laboratory of Prof. Bruce Gale (Department of Engineering, University of Utah). The high density production of lipid arrays has potential applications in many fields such as biosensing, drug discovery, proteomics and clinical diagnostics.
Label-Free Detection of Proteins and Small-Moleclues:
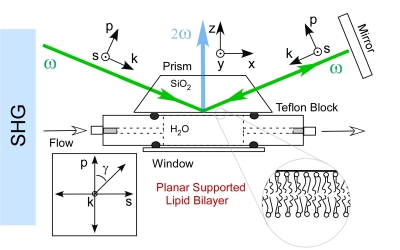 Analytical methods which can quantify the interaction of proteins and low molecular
weight drug compounds with biological membranes in a label-free manner are needed
to address a growing number of questions in the areas of pharmaceutics and biotechnology.
What is needed is a noninvasive method to detect drug and protein association to the
lipid membrane without the use of an extrinsic label. The nonlinear technique of second
harmonic generation (SHG)may hold the answer to this problem.
Analytical methods which can quantify the interaction of proteins and low molecular
weight drug compounds with biological membranes in a label-free manner are needed
to address a growing number of questions in the areas of pharmaceutics and biotechnology.
What is needed is a noninvasive method to detect drug and protein association to the
lipid membrane without the use of an extrinsic label. The nonlinear technique of second
harmonic generation (SHG)may hold the answer to this problem.
We have successfully demonstrated thatSHG is a viable tool for label-free detection
of protein adsorption to biological membranes. Our future goals are to expand the
use of SHG and related nonlinear optical methods for the detection not only of proteins
but small molecule-drugs with biological membranes. These stu dies are being coupled with our capabilities to produce multi-component lipid bilayer
arrays (as discussed above) to provide an efficient high-throughput, label-free assay
for the interrogation of protein and small molecule interactions with membranes. The
practical implementation of these technologies will have a significant impact on the
pharmacological screening of drug candidates, and the investigation of drug-membrane
interactions.
dies are being coupled with our capabilities to produce multi-component lipid bilayer
arrays (as discussed above) to provide an efficient high-throughput, label-free assay
for the interrogation of protein and small molecule interactions with membranes. The
practical implementation of these technologies will have a significant impact on the
pharmacological screening of drug candidates, and the investigation of drug-membrane
interactions.
Lipid Bilayer Structure and Dynamics:
We are also pursuing research aimed at understanding the complex interplay between
themovement of lipid species across the cellular membrane and the establishment of
lipid compositional asymmetry. A full understanding of the mechanism by which  membrane asymmetry is achieved and maintained in cellular systems has not been realized
to date; primarily due to the difficultyof studying membrane biophysical phenomena
in a non-destructive or non-perturbing fashion. It has been suggested that lipid membrane
asymmetry is maintained by unidirectional lipid transporters, in conjunction with
a high energetic barrier to translocation which limits the rate at which lipids might
spontaneously translocate across the membrane. However, a growing number of publications
demonstrate cases of rapid spontaneous translocation of phospholipids.
membrane asymmetry is achieved and maintained in cellular systems has not been realized
to date; primarily due to the difficultyof studying membrane biophysical phenomena
in a non-destructive or non-perturbing fashion. It has been suggested that lipid membrane
asymmetry is maintained by unidirectional lipid transporters, in conjunction with
a high energetic barrier to translocation which limits the rate at which lipids might
spontaneously translocate across the membrane. However, a growing number of publications
demonstrate cases of rapid spontaneous translocation of phospholipids.
The connection between lipid compositional asymmetry and flip-flop in planar supported
lipid bilayers is being explored using a novel application of sum-frequency vibrational
spectroscopy (SFVS) developed by our groupto selectively probe the asymmetry in a
planar-supported lipid bilayer (PSLB). This new surface analytical method allows for
the direct detection of lipid flip-flop without 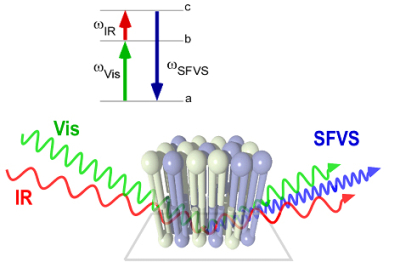 the need for a fluorescent or spin-labeled lipid probe, which can alter the measured
translocation rates. The goal of this research is to use this surface analytical tool
to address some of the central issues concerning the transbilayer movement and establishment
of lipid asymmetry in bilayer systems. Our work is attempting to address the effect
of phospholipid fatty acid alkyl chain length and saturation on the rate of flip-flop,
and the effect of headgroup chemistry and charge on lipid migration. In addition,
the coupling of lipid flip-flop energetics to the establishment of lipid asymmetry
is also being explored. Theses studies are aimed at providing physical insight into
the mechanism of lipid compositional asymmetry.
the need for a fluorescent or spin-labeled lipid probe, which can alter the measured
translocation rates. The goal of this research is to use this surface analytical tool
to address some of the central issues concerning the transbilayer movement and establishment
of lipid asymmetry in bilayer systems. Our work is attempting to address the effect
of phospholipid fatty acid alkyl chain length and saturation on the rate of flip-flop,
and the effect of headgroup chemistry and charge on lipid migration. In addition,
the coupling of lipid flip-flop energetics to the establishment of lipid asymmetry
is also being explored. Theses studies are aimed at providing physical insight into
the mechanism of lipid compositional asymmetry.

