Size-Selected Cluster Deposition and Model Catalyst Studies
The problem:
Many important catalysts consist of small clusters or nanoparticles of active metals
or metal oxides, dispersed on a high surface area support (e.g. titania, alumina,
silica). These catalysts are complex and difficult to study, therefore many groups
have turned to planar model catalysts where catalytic clusters/nanoparticles are grown
on a single crystal or thin film support, allowing them to be studied by surface science
methods like STM, ion scattering, electron spectroscopies, and a variety of mass spectrometry
methods. In such experiments, there is still a rather broad range of cluster/nanoparticle
sizes present, as well as distributions of metal-support geometries. In our experiments,
we grow catalytic cluster ions in the gas phase, then use a mass spectrometer to select
a particular size/composition of interest, which is then deposited on a single crystal
support to make a truly monodisperse model catalyst. We then use a variety of in situ
methods to characterize morphology, electronic structure, adsorbate binding, and catalytic
activity. Typical results are summarized in the PDF files linked below.
 Funding AFOSR and DOE
Funding AFOSR and DOE
Recent Results: (Note: the slide shows may be too big if you have a slow internet connection).
CO oxidation on Aun on TiO2
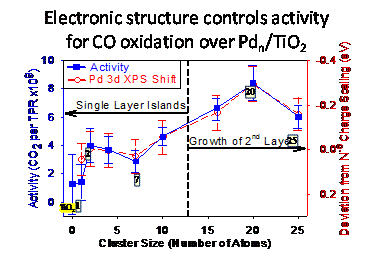
CO oxidation over Pd/TiO2 - correlations of activity with electronic structure and adsorbate binding
